Optimization of Ultrasound-Assisted Cold-Brew Method for Developing Roselle (Hibiscus sabdariffa L.)-Based Tisane with High Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Plant Materials
2.3. Ultrasound-Assisted Extraction
2.4. Analysis of the Phenolic by HPLC-DAD
2.5. Analysis of the Phenolic Stability by HPLC-DAD
2.6. Evaluation of the Extraction Process
2.7. DPPH Assay
2.8. FRAP Assay
2.9. ABTS Assay
2.10. Experimental Design and Data Analysis
3. Results and Discussion
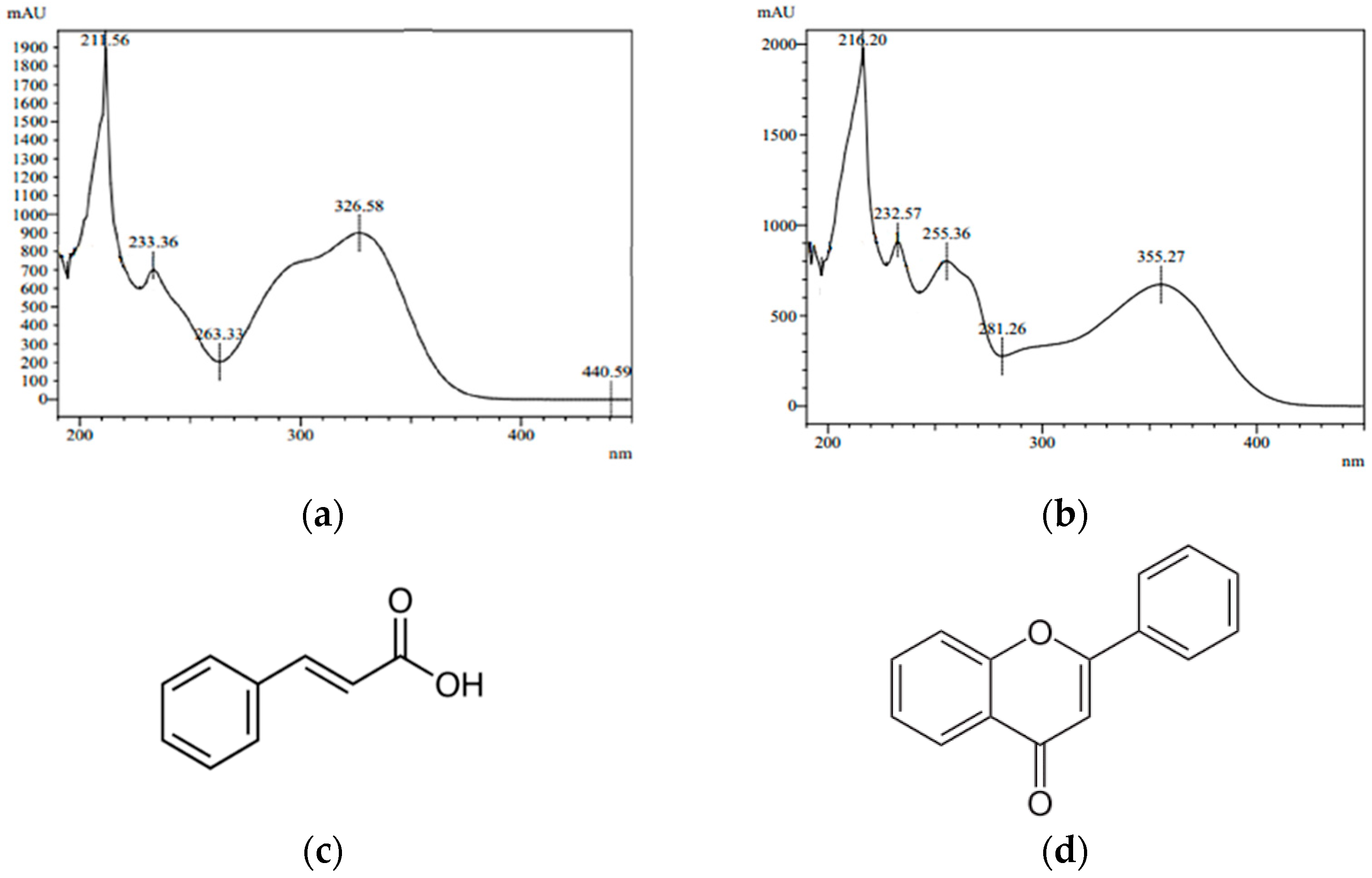
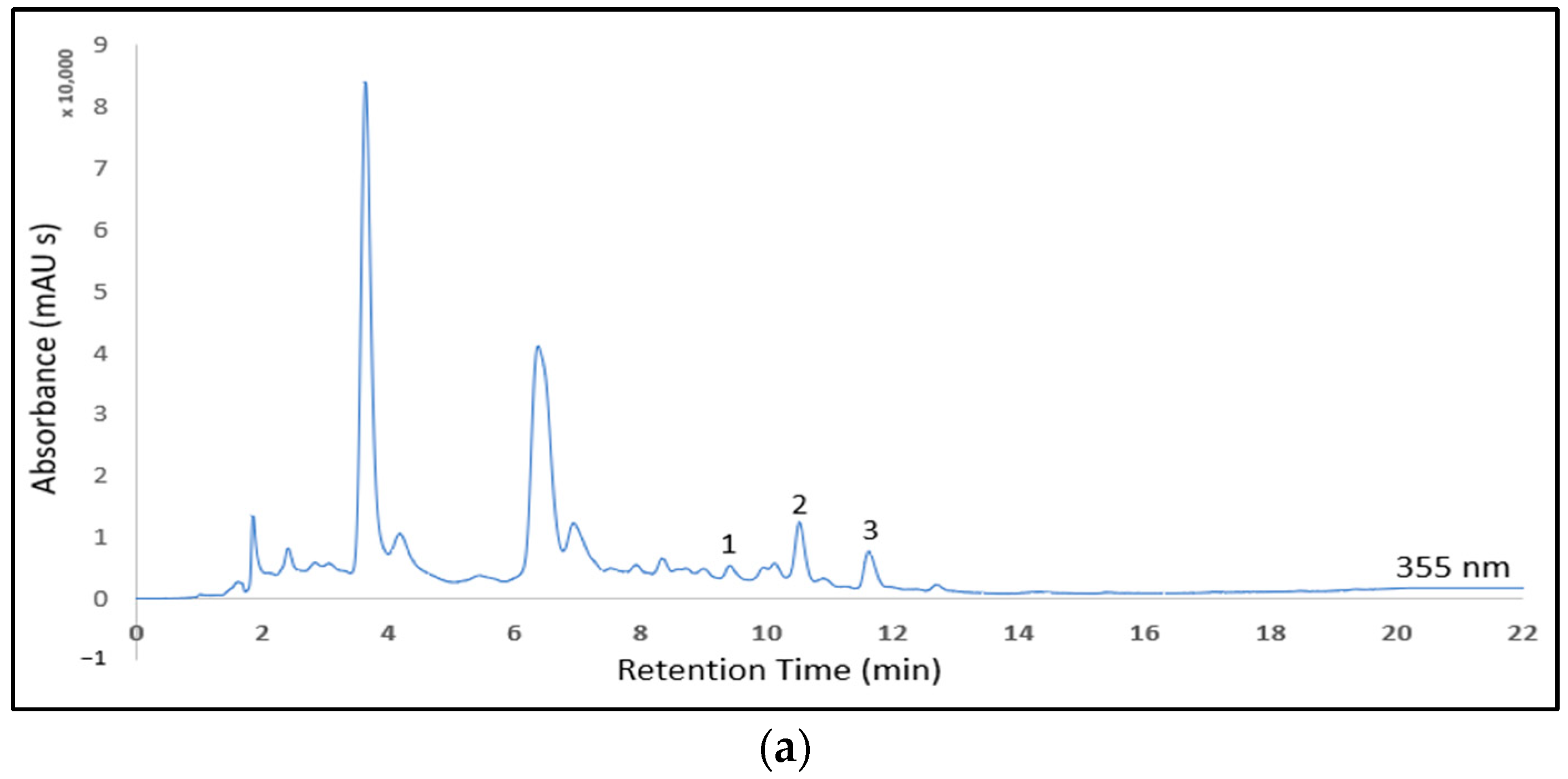
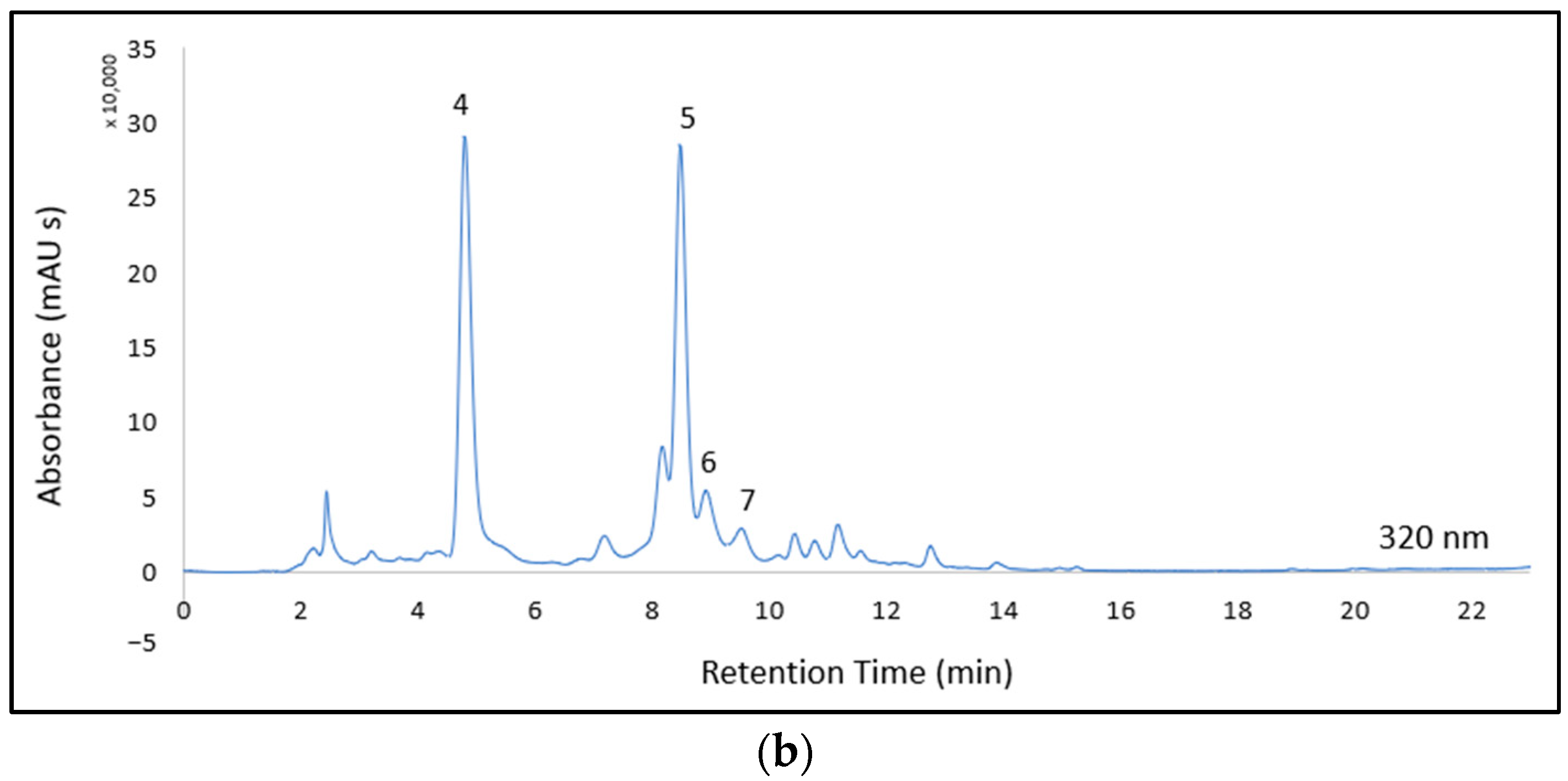
3.1. HPLC Performance for Analysis of Phenolic Compound
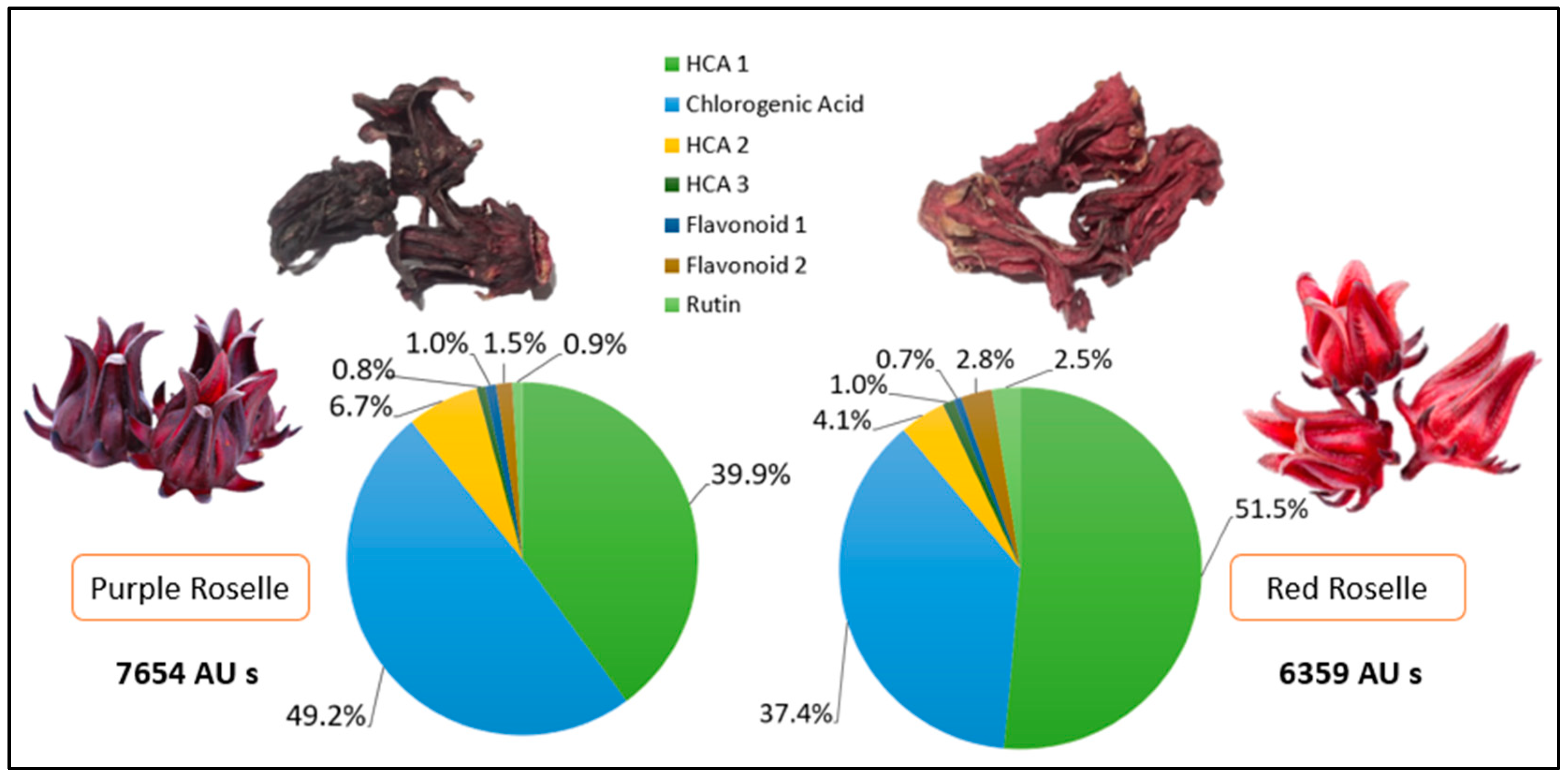
3.2. Phenolic Profile of Roselle Tisane
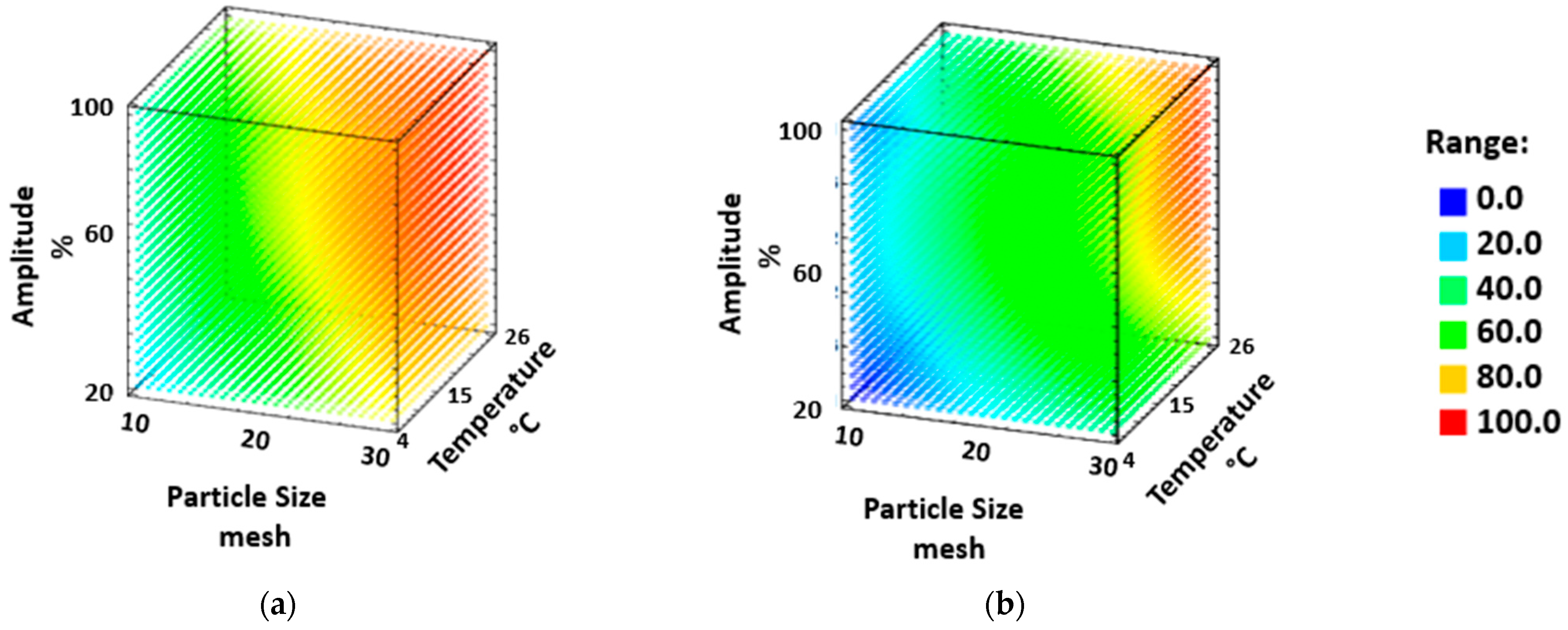
3.3. Optimization of UAE Method
3.4. Extraction Process Evaluation
3.5. Roselle Tisane Production
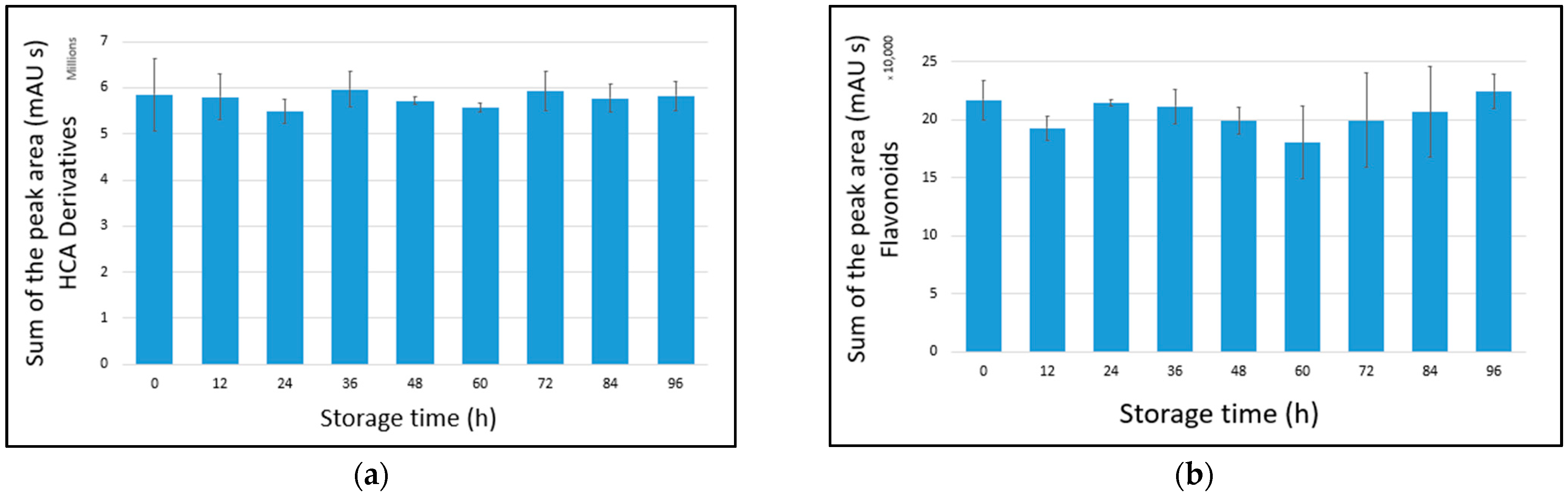
3.6. Phenolic Stability
3.7. Antioxidant Activity Characteristic
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales-de la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Application of Novel Processing Methods for Greater Retention of Functional Compounds in Fruit-Based Beverages. Beverages 2016, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Reis Giada, M.d.L. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; InTech: London, UK, 2013. [Google Scholar]
- Chang, H.P.; Ma, C.C.; Chen, H.S. The Impacts of Young Consumers’ Health Values on Functional Beverages Purchase Intentions. Int. J. Environ. Res. Public Health 2020, 17, 3479. [Google Scholar] [CrossRef] [PubMed]
- Hapsari, B.W.; Manikharda; Setyaningsih, W. Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health. Horticulturae 2021, 7, 35. [Google Scholar] [CrossRef]
- Osei-Kwarteng, M.; Gweyi-Onyango, J.P.; Mahunu, G.K. Harvesting, Storage, Postharvest Management, and Marketing of Hibiscus sabdariffa. In Roselle (Hibiscus sabdariffa): Chemistry, Production, Products, and Utilization; Elsevier: London, UK, 2021; pp. 15–31. ISBN 9780128221006. [Google Scholar]
- Osei-Kwarteng, M.; Gweyi-Onyango, J.P.; Mahunu, G.K. Harvesting, Storage, Postharvest Management, and Marketing of Hibiscus sabdariffa. In Roselle (Hibiscus sabdariffa); Breeding, genetic diversity, and safe production of Hibiscus sabdariffa under climate change; Elsevier: London, UK, 2021; pp. 1–10. ISBN 9780128221006. [Google Scholar]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Stability of 40 Phenolic Compounds during Ultrasound-Assisted Extractions (UAE). In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2016; Volume 1755. [Google Scholar]
- Müller, A.L.H.; De Oliveira, J.A.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Design of Experiments and Method Development. In Solid-Phase Extraction; Elsevier: London, UK, 2019; pp. 589–608. ISBN 9780128169063. [Google Scholar]
- Damiani, E.; Bacchetti, T.; Padella, L.; Tiano, L.; Carloni, P. Antioxidant Activity of Different White Teas: Comparison of Hot and Cold Tea Infusions. J. Food Compos. Anal. 2014, 33, 59–66. [Google Scholar] [CrossRef]
- Willaert, R.G.; Baron, G. Wort Boiling Today-Boiling Systems with Low Thermal Stress in Combination with Volatile Stripping. Belgian J. Brewing Biotechnol. 2021, 26, 217–230. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of Grinding, Extraction Time and Type of Coffee on the Physicochemical and Flavour Characteristics of Cold Brew Coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef] [Green Version]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and Antioxidative Properties of Red and Black Rice Varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Assefa, A.D.; Saini, R.K.; Keum, Y.S. Extraction of Antioxidants and Flavonoids from Yuzu (Citrus Junos Sieb Ex Tanaka) Peels: A Response Surface Methodology Study. J. Food Meas. Charact. 2017, 11, 364–379. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Original Contribution Antioxidant Activity Applying an Improved ABTS Radical Cation. Free Radical Biology Med. 1999, 26. [Google Scholar] [CrossRef]
- ICH. ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology; European Medicines Agency: Amsterdam, The Nertherlands, 1995.
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Salum, M.L.; Arroyo Mañez, P.; Luque, F.J.; Erra-Balsells, R. Combined Experimental and Computational Investigation of the Absorption Spectra of E- and Z-Cinnamic Acids in Solution: The Peculiarity of Z-Cinnamics. J. Photochem. Photobiol. B 2015, 148, 128–135. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant Capacities and Total Phenolic Contents of 30 Flowers. Ind. Crops Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Fathimah, R.N.; Setyaningsih, W.; Carrera, C.; Palma, M. Microwave-Assisted Extraction of Hibiscus sabdariffa Antioxidants: Method Development and Validation. Proceedings 2020, 70, 7681. [Google Scholar] [CrossRef]
- Jaya Prakkash, M.A.; Ragunathan, R.; Jesteena, J. Evaluation of Bioactive Compounds from Jasminum Polyanthum and Its Medicinal Properties. J. Drug Deliv. Ther. 2019, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Zannou, O.; Kelebek, H.; Selli, S. Elucidation of Key Odorants in Beninese Roselle (Hibiscus sabdariffa L.) Infusions Prepared by Hot and Cold Brewing. Food Res. Int. 2020, 133, 109133. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible Flowers: Review of Flower Processing and Extraction of Bioactive Compounds by Novel Technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef]
- Xu, D.P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.N.; Li, H. Bin Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Jatropha Integerrima by Response Surface Methodology. Molecules 2016, 21, 18. [Google Scholar] [CrossRef] [Green Version]
- Mota, F.L.; Carneiro, A.P.; Queimada, A.J.; Pinho, S.P.; Macedo, E.A. Temperature and Solvent Effects in the Solubility of Some Pharmaceutical Compounds: Measurements and Modeling Temperature and Solvent Effects in the Solubility of Some Pharmaceutical Compounds: Measure-Ments and Modeling. Eur. J. Pharm. Sci. 2009, 37, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyroglou, S.; Laskari, R.; Vareltzis, P. Optimization of Sensory Properties of Cold Brew Coffee Produced by Reduced Pressure Cycles and Its Physicochemical Characteristics. Molecules 2022, 27, 2971. [Google Scholar] [CrossRef] [PubMed]
- Tarasevičienė, Ž.; Vitkauskaitė, M.; Paulauskienė, A.; Černiauskienė, J. Wild Stinging Nettle (Urtica Dioica L.) Leaves and Roots Chemical Composition and Phenols Extraction. Plants 2023, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.; Pinho, S.P. Solubility of Flavonoids in Pure Solvents. Ind. Eng. Chem. Res. 2012, 51, 6586–6590. [Google Scholar] [CrossRef]
- Christian, K.R.; Jackson, J.C. Changes in Total Phenolic and Monomeric Anthocyanin Composition and Antioxidant Activity of Three Varieties of Sorrel (Hibiscus sabdariffa) during Maturity. J. Food Compos. Anal. 2009, 22, 663–667. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An Outlook on Chlorogenic Acids-Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Trinh, L.T.P.; Choi, Y.S.; Bae, H.J. Production of Phenolic Compounds and Biosugars from Flower Resources via Several Extraction Processes. Ind. Crops Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Antioxidant Activity and Stability of Natural Colour Recovered from Roselle Juice Production. Agro Food Industry Hi Tech 2013, 24, 40–43. [Google Scholar]
- Samadi, S.; Raouf Fard, F. Phytochemical Properties, Antioxidant Activity and Mineral Content (Fe, Zn and Cu) in Iranian Produced Black Tea, Green Tea and Roselle Calyces. Biocatal. Agric. Biotechnol. 2020, 23, 101472. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility of Gallic Acid, Catechin, and Protocatechuic Acid in Subcritical Water from (298.75 to 415.85) K. J. Chem. Eng. Data 2010, 55, 3101–3108. [Google Scholar] [CrossRef]
- Kwok, R.; Lee Wee Ting, K.; Schwarz, S.; Claassen, L.; Lachenmeier, D.W. Current Challenges of Cold Brew Coffee—Roasting, Extraction, Flavor Profile, Contamination, and Food Safety. Challenges 2020, 11, 26. [Google Scholar] [CrossRef]
- Deng, M.; Deng, Y.; Dong, L.; Ma, Y.; Liu, L.; Huang, F.; Wei, Z.; Zhang, Y.; Zhang, M.; Zhang, R. Effect of Storage Conditions on Phenolic Profiles and Antioxidant Activity of Litchi Pericarp. Molecules 2018, 23, 2276. [Google Scholar] [CrossRef] [Green Version]
- Padda, M.S.; Picha, D.H. Effect of Low Temperature Storage on Phenolic Composition and Antioxidant Activity of Sweetpotatoes. Postharvest Biol. Technol. 2008, 47, 176–180. [Google Scholar] [CrossRef]
- Chang, M.Y.; Lin, Y.Y.; Chang, Y.C.; Huang, W.Y.; Lin, W.S.; Chen, C.Y.; Huang, S.L.; Lin, Y.S. Effects of Infusion and Storage on Antioxidant Activity and Total Phenolic Content of Black Tea. Appl. Sci. 2020, 10, 2685. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, S.; Xu, T.; Xieli, C. Study on the Stability of the Effective Components Cinnamic Acid in the Decoction of Cinnamon. J. Pharm. Pract. 2020, 38, 255–258. [Google Scholar]
- Van Der Sluis, A.A.; Dekker, M.; Van Boekel, M.A.J.S. Activity and Concentration of Polyphenolic Antioxidants in Apple Juice. 3. Stability during Storage. J. Agric. Food Chem. 2005, 53, 1073–1080. [Google Scholar] [CrossRef]
- Lyu, J.I.; Kim, J.M.; Kim, D.G.; Kim, J.B.; Kim, S.H.; Ahn, J.W.; Kang, S.Y.; Ryu, J.; Kwon, S.J. Phenolic Compound Content of Leaf Extracts from Different Roselle (Hibiscus sabdariffa) Accessions. Plant Breed. Biotechnol. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Morales-Cabrera, M.; Hernández-Morales, J.; Leyva-Rúelas, G.; Salinas-Moreno, Y.; Soto-Rojas, L.; Castro-Rosas, J. Influence of Variety and Extraction Solvent on Antibacterial Activity of Roselle (Hibiscus sabdariffa L.) Calyxes. J. Med. Plants Res. 2013, 7, 2319–2322. [Google Scholar] [CrossRef] [Green Version]
- Larrauri, A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]


| Run | Factors | * Response (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | HCA Derivatives | Flavonoid Derivatives | |||||
| Observed | Predicted | Error | Observed | Predicted | Error | |||||
| 1 | 1 | 1 | 0 | 0 | 100.00 | 96.51 | 3.49 | 100.00 | 93.39 | 6.61 |
| 2 | 0 | 0 | 0 | 0 | 75.70 | 76.31 | 0.80 | 60.45 | 56.04 | 7.30 |
| 3 | 0 | 1 | 1 | 0 | 77.91 | 80.91 | 3.85 | 57.32 | 63.20 | 10.26 |
| 4 | 1 | 0 | −1 | 0 | 87.49 | 87.76 | 0.31 | 75.72 | 69.85 | 7.76 |
| 5 | −1 | 0 | 1 | 0 | 56.43 | 55.46 | 1.71 | 20.70 | 26.50 | 28.04 |
| 6 | 0 | −1 | −1 | 0 | 53.82 | 50.84 | 5.52 | 34.41 | 29.48 | 14.31 |
| 7 | 0 | 1 | 0 | −1 | 71.86 | 64.91 | 9.66 | 52.14 | 40.55 | 22.23 |
| 8 | 0 | −1 | 1 | 0 | 74.77 | 71.07 | 4.95 | 54.57 | 46.71 | 14.41 |
| 9 | 1 | 0 | 0 | −1 | 75.71 | 79.55 | 5.08 | 46.44 | 53.99 | 16.27 |
| 10 | 0 | 0 | −1 | −1 | 43.91 | 46.51 | 5.93 | 17.30 | 25.01 | 44.57 |
| 11 | 0 | 1 | −1 | 0 | 73.06 | 76.79 | 5.10 | 56.00 | 64.82 | 15.74 |
| 12 | 0 | 0 | 0 | 0 | 73.17 | 76.31 | 4.30 | 46.23 | 56.04 | 21.22 |
| 13 | 0 | 0 | −1 | 1 | 72.25 | 67.40 | 6.71 | 48.76 | 42.48 | 12.89 |
| 14 | 0 | 0 | 1 | 1 | 76.02 | 74.09 | 2.54 | 60.64 | 52.05 | 14.17 |
| 15 | 0 | 1 | 0 | 1 | 82.33 | 84.53 | 2.67 | 65.61 | 67.63 | 3.07 |
| 16 | 1 | 0 | 1 | 0 | 91.61 | 89.68 | 2.11 | 78.51 | 77.88 | 0.81 |
| 17 | −1 | −1 | 0 | 0 | 29.98 | 34.14 | 13.89 | 10.59 | 16.32 | 54.10 |
| 18 | 0 | −1 | 0 | 1 | 56.18 | 62.43 | 11.12 | 22.36 | 33.88 | 51.49 |
| 19 | −1 | 0 | −1 | 0 | 31.80 | 33.03 | 3.86 | 18.36 | 18.92 | 3.07 |
| 20 | −1 | 0 | 0 | 1 | 54.30 | 50.48 | 7.03 | 28.68 | 22.08 | 23.02 |
| 21 | 1 | −1 | 0 | 0 | 87.24 | 86.41 | 0.95 | 63.68 | 61.31 | 3.72 |
| 22 | −1 | 0 | 0 | −1 | 31.87 | 29.76 | 6.63 | 10.48 | 3.50 | 66.63 |
| 23 | 0 | −1 | 0 | −1 | 54.13 | 51.23 | 5.36 | 24.56 | 22.47 | 8.49 |
| 24 | −1 | 1 | 0 | 0 | 58.32 | 59.82 | 2.57 | 34.58 | 36.07 | 4.30 |
| 25 | 1 | 0 | 0 | 1 | 87.51 | 89.65 | 2.44 | 65.95 | 73.89 | 12.03 |
| 26 | 0 | 0 | 0 | 0 | 80.07 | 76.31 | 4.69 | 61.43 | 56.04 | 8.79 |
| 27 | 0 | 0 | 1 | −1 | 58.64 | 64.16 | 9.41 | 25.64 | 31.04 | 21.07 |
| Phenolic Compound | Linear Equation | LOD (µg mL−1) | LOQ (µg mL−1) | |||
|---|---|---|---|---|---|---|
| High Range | R2 | Low Range | R2 | |||
| Chlorogenic Acid | y = 54,048x + 26,370 | 0.999 | y = 54,147x − 302 | 0.999 | 0.24 | 0.73 |
| Rutin | y = 35,773x + 21,028 | 0.999 | y = 37,588x − 1154 | 0.997 | 0.71 | 2.15 |
| Phenolic Compound | Precision CV (%) | |
|---|---|---|
| Repeatability | Intermediate Precision | |
| HCA 1 | 1.99 | 3.19 |
| Chlorogenic acid | 2.34 | 6.13 |
| HCA 2 | 9.91 | 8.13 |
| HCA 3 | 3.69 | 7.06 |
| Flavonoid 1 | 8.54 | 8.46 |
| Flavonoid 2 | 2.78 | 6.78 |
| Rutin | 2.93 | 7.80 |
| Phenolic Compounds | Roselle Tisane Sample (mg L−1) | |
|---|---|---|
| Purple | Red | |
| Chlorogenic Acid | 69.22 ± 1.67 | 43.48 ± 0.39 |
| Rutin | 2.13 ± 0.06 * | 4.58 ± 0.20 |
| Antioxidant Activity Assay | IC50 Value (µg mL−1) | ||||
|---|---|---|---|---|---|
| Ascorbic Acid | Conventional | UAE | |||
| Day 1 | Day 5 | Day 1 | Day 5 | ||
| DPPH | 3.23 | 9.77 ± 0.01 a | 10.75 ± 0.04 b | 9.54 ± 0.06 a | 9.57 ± 0.01 a |
| ABTS | 3.58 | 8.06 ± 0.02 a | 8.69 ± 0.10 b | 7.80 ± 0.03 a | 7.82 ± 0.14 a |
| FRAP | 6.99 | 10.34 ± 0.03 a | 10.88 ± 0.11 b | 9.96 ± 0.01 a | 10.01 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larasati, I.D.; Oktaviani, N.M.D.; Lioe, H.N.; Estiasih, T.; Palma, M.; Setyaningsih, W. Optimization of Ultrasound-Assisted Cold-Brew Method for Developing Roselle (Hibiscus sabdariffa L.)-Based Tisane with High Antioxidant Activity. Beverages 2023, 9, 58. https://doi.org/10.3390/beverages9030058
Larasati ID, Oktaviani NMD, Lioe HN, Estiasih T, Palma M, Setyaningsih W. Optimization of Ultrasound-Assisted Cold-Brew Method for Developing Roselle (Hibiscus sabdariffa L.)-Based Tisane with High Antioxidant Activity. Beverages. 2023; 9(3):58. https://doi.org/10.3390/beverages9030058
Chicago/Turabian StyleLarasati, Intan Dewi, Nurul Mutmainah Diah Oktaviani, Hanifah Nuryani Lioe, Teti Estiasih, Miguel Palma, and Widiastuti Setyaningsih. 2023. "Optimization of Ultrasound-Assisted Cold-Brew Method for Developing Roselle (Hibiscus sabdariffa L.)-Based Tisane with High Antioxidant Activity" Beverages 9, no. 3: 58. https://doi.org/10.3390/beverages9030058







